- Here we present highlights from a recent Biostime Nutrition webinar with guest speaker Dr Paula Smith-Brown. Paula is a PhD qualified accredited practising dietitian, with a specialist interest in young child nutrition, food allergy and intolerance, and the microbiome.

There is abundant evidence that immune disorders are increasing worldwide in infants and children. Prescott and colleagues published a global survey of changing patterns of food allergy burden in children in 2013, which demonstrated that children under the age of 5 years food allergy prevalence based on oral food challenges, ranged from 1% in data collected in Thailand to 10% in children from Australia.1
Dr Paula Smith-Brown received a speaker honorarium from Biostime® Nutrition. The summary was written by Professor Peter SW Davies, Honorary Professor of Childhood Nutrition, University of Queensland. Prof Davies received a writing honorarium from Biostime® Nutrition.
The first 1000 days (from conception to around 2 years of age) has been identified as a critical window of opportunity to influence long-term health, including immune disorders.2,3 This period is associated with the establishment of both the gut microbiome and lifelong eating habits.4,5
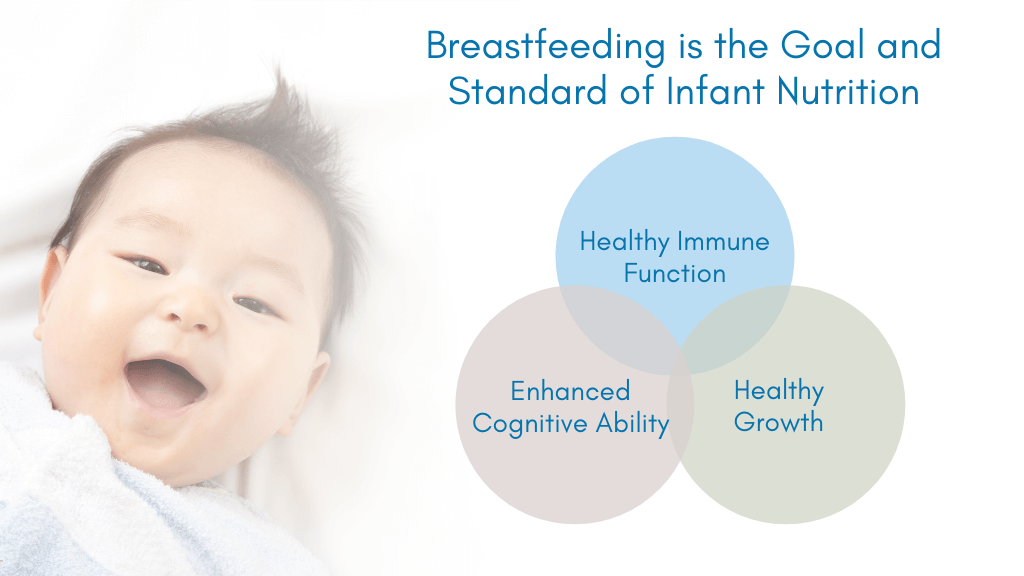
Clearly diet is of crucial importance at this time, international and national recommendations state that infants should be exclusively breast-fed up to around 6 months of age, but importantly that breastfeeding should continue until 2 years of age or beyond, for as long as the mother and child desire.6,7
There are a number of components of breast milk, that are of importance to the development of immune system at this time, including the microbiome of breast milk itself, human milk oligosaccharides (HMOs), and secretory IgA.
Breast milk contains a microbiome which serves as an ongoing source of potentially colonising bacteria to infants and children.8–10 The composition of the microbiome is influenced by several factors, such as maternal weight and mode of delivery.11
The human milk microbiome changes during lactation, and milk from obese mothers tends to contain a different and less diverse bacterial community, compared with normal weight mothers. It has also been demonstrated that milk samples from mothers undergoing elective (but not non-elective) caesarean delivery contained a different bacterial profile to mothers who gave birth by vaginal delivery. It was suggested that differences in physiological stress or hormonal signals during elective caesarean section delivery might account for these diferences.11
Recently, the strength of the relationship between mode of delivery and breast milk microbiome was further strengthened by evidence from a study in Finland.12
In this study, both the mode of delivery and intrapartum antibiotic exposure were significantly associated with changes in milk microbial composition, however, mode of delivery had a more profound effect on the microbiome. Bifidobacterium species were uniquely found in the breast milk of mothers who did not receive intrapartum antibiotics. It was suggested that the dysbiosis found in infants born by caesarean section might be exacerbated by the changes in breast milk microbiome.13,14
The infants developing immune system is supported by passive immunity. During the last trimester of pregnancy maternal IgG crosses the placenta until birth. After that time, passive immunity is supported by breast milk, which contains between 0.4 to 1.0 g/L of secretory IgA.15 Planer and colleagues, showed in a complex study using both animal and human models, that the specific bacterium targeted by IgA changed throughout the first 2 years of life.
Breastfeeding associated bacteria, Bifidobacterium longum, was rarely targeted in the first year of life, while the more commonly pathogenic bacteria, Escherichia Coli, was targeted strongly throughout the first 2 years of life.16 Dzidic and colleagues showed that alterations in IgA targeting of the microbiome in infancy preceded the development of allergy in 7-year-old children.17 The children with allergies (particularly asthma) had a lower proportion of IgA bound faecal bacteria at 12 months of age, compared with healthy children.
Moreover, the bacterial targets of early IgA responses differed between healthy children and those who later developed allergies. They concluded that an aberrant IgA response to the gut microbiome during infancy precedes asthma and allergy development.

Development of serum immunoglobulins in early life. Secretory IgA delivered via breastfeeding remains confined to the intestine and does not contribute to IgA levels in serum.15
These multifunctional glycans are the third most abundant solid component of human milk after lactose and lipids.18 They are made of five basic monosaccharides, namely glucose, galactose, N-Acetylglucosamine, fucose and sialic acid. The different structures seem to influence the functioning of the 150 to 200 HMOs that have been identified to date.
HMOs act as a powerful prebiotic in humans and are digested by potentially beneficial bacteria in the large intestine notably the infant microbiome associated Bifidobacterium species.19 HMOs also directly influence the functioning of the immune system. For example, HMOs are known to reduce intestinal crypt cell proliferation, increase barrier function and inhibit infections by bacteria and viruses, by binding to pathogens in the gut lumen or inhibiting binding to cell surface glycan receptors.20,21
A number of studies have assessed the potential benefits of adding HMOs to infant formula.22–26 Puccio and colleagues, showed that the addition of two HMOs to infant formula was safe, well tolerated and supported age-appropriate growth.25 Moreover, there was a significant reduction in bronchitis in the first year of life, in infants fed formula supplemented with HMOs when compared to infants fed standard formula.
The amounts of HMOs in human milk varies markedly between women, although the HMO profile of any one individual is much more constant. There are many factors that affect or drive the HMOs produced by individual women. Notably, an important factor relates to the Lewis system and secretor status.27 Azad and colleagues28 reported seasonal and parity effects, whilst others have shown geographic effects29 and animal studies indicate that diet and exercise can change the amount and profile of HMOs.30 Recently Quin and colleagues found that maternal diets high in fruit and unsaturated fatty acids were positively correlated with an increased absolute abundance of many HMOs, whilst cured meat intake had a negative effect.31
Human genes are required to express the enzymes that create links between monosaccharide building blocks to create HMOs. The Secretor or FUT2 gene encodes an enzyme required to synthesise any HMO with an -1-2-fucose bond, such as the HMO, 2’-fucosyllactose (2’FL).
However, about 20% of individuals have an inactive FUT2 or secretor gene, thus only around 70–80% of breast milk samples contain 2’-FL.32 The concentration of this HMO varies significantly during lactation and between mothers.33–35 There seems to be significant geographic variability between so called secretor and non-secretor mothers.
Castanys-Munoz and colleagues32 showed that the percentage of mothers who had 2’-FL in their breast milk varied markedly, from 100% in samples collected in Mexico to 46% in samples collected from the Philippines.
Infant associated Bifidobacterium species contain genes to digest the -1-2 fucose bond, which is missing from HMOs found in the breast milk of non-secretor mothers.36 It should therefore not be a surprise that the microbiome of breast-fed infants also seems to vary based on the mother’s secretor status. One of the earliest studies in this area clearly showed that on average potentially beneficial Bifidobacterium were established earlier and more often in infants fed by secretor mothers than in infants fed by non-secretor mothers.37 Importantly, it has also been shown that the influence of non-secretor breast milk lasts even after breastfeeding has ceased.
In a pilot study, children aged 2–3 years of age, who had received non-secretor breast milk had lower levels of Bifidobacterium compared with children who had received secretor breast milk.38
This is important as it has recently been shown that low gut Bifidobacterium abundance is associated with markers of intestinal inflammation.39 Moreover, feeding B. infantis EVC001 silenced intestinal Th2 and Th17 and upregulated IFNb, while faecal water from infants fed this probiotic skewed naïve T-cell polarization towards Th1 and away from Th2, suggesting that metabolites of this bacteria had the capacity to support healthier immune imprinting during the first critical months of life.

Comparison of relative levels of gut microbiota in secretor-fed infants and non-secretor-fed infants.38
*Indicates significant differences (p<0.05) in the relative levels of various gut microbes using a Wilcoxon rank sum test. The colour boxplots show the quartiles above and below the median; the dark line near the centre of the box denotes the median. The whiskers extend to the first and fourth quartiles, and the black dots show outliers.
N=Non-secretor, S=Secretor.
Breast milk plays an important role in the developing immune system, via several factors, including the provision of secretory IgAs and HMOs, and the effect of these HMOs on the developing microbiome. These data strongly support international and national recommendations stating that infants should be exclusively breast fed to 6 months6 or to around 6 months of age7 but importantly that breastfeeding should continue until 2 years of age or beyond for as long as the mother and child desire.
-
Prescott SL, et al. WAO J 2013;6:21.
-
Davies PSW, et al. J Dev Orig Health Dis 2016;7:1–9.
-
Bryce J, et al. Lancet 2008;371:510–26.
-
Yatsunenko T, et al. Nature 2012;486:222–7.
-
Skinner JD, et al. J Am Diet Assoc 2002;102:1638–47
-
World Health Organisation. Health Topics – Breastfeeding. Available at: https://www.who.int/health topics/breastfeeding#tab=tab_2.
-
NHMRC. 2012. Infant Feeding Guidelines. Canberra. NHMRC. Available at: https://www.nhmrc.gov.au/about us/publications/infant-feeding-guidelines-information-health-workers.
-
Jost T, et al. Environ Microbiol 2014;16:2891–2904.
-
Kumar H, et al. Front Microbiol 2016;7:1619
-
Pannaraj PS, et al. JAMA Pediatr 2017;647–54.
-
Cabrera-Rubio R, et al. Am J Clin Nutr 2012; 96:544–51.
-
Hermansson H, et al. Front Nutr 6.4. doi:10.3389/fnut.2019.00004.
-
Shao Y, et al. Nature 2019; Doi.org/10.1038/s41586-019-1560-1.
-
Hoang DM, et al. Acta Paediatrica Doi: 10.1111/apa.15501.
-
Niers L, et al. Nutrition Reviews 2007;65(8): 347–60.
-
Planer JD, et al. Nature 2016:263–6.
-
Dzidic M, et al. J Allergy Clin Immunol 2017;139:1017–25.
-
Bode L. Glycobiology 2012;22(9):1147–62.
-
Holman RC, et al. Paeditr Perinat Epidemol 2006; 20:498–506.
-
Donovan SM and Comstock SS. Ann Nutr Metab 2016;432:62–70.
-
Zuurveld M, et al. Front Immunol 2020;11.801.Doi:10.3389/ fimmu.2020.00801.
-
Marriage BJ, et al. JPGN 2015;61(6):649–58.
-
Berger B, et al. Eur J Pediatr 175;1505.
-
Goehring KC, et al J Nutr 2016;146:2559–66.
-
Puccio G, et al. JPGN 2017;64(4):524–631
-
Nowak-Wegrzn A, et al. Nutrients 2019;11:1447.
-
Bode L, et al. Science 2020;367:6482.
-
Azad MB, et al. J Nutr 2018;148: 1733–42.
-
McGuire MK, et al. Am J Clin Nutr 2017;105:1086–1100.
-
Harris JE, et al. Nature Metabolism 2020;2:678–87.
-
Quien C, et al. J Biol Chem 2020;295(12):4035–48.
-
Castanys-Munboz E, et al. Nutr Revs 2013;71(12)773–89
-
Chaturvedi P, et al. Glcobiology 2001;11:365–72.
-
Erney RM, et al. JPGN 2000;30:181–92.
-
Thurl S, et al. Br J Nutr 2010; 104:1261–71.
-
Sakanaka. Nutrients 2019;12(1):71.doi: 10.3390/nu12010071
-
Lewis ZT, et al. Microbiome 2015;3:13.
-
Smith-Brown P, et al. PLOS One 2016;Doi:10.1371/journal.pone.161221
-
Henrick, et al. 2020;https://doi.org/10.1101/2020.10.24.353250
Are you a Healthcare Professional?
Important Notice and Declaration
Breast milk is best for babies. Professional advice should be followed before using an infant formula. Introducing partial bottle feeding could negatively affect breast feeding. Good maternal nutrition is important for breast feeding and reversing a decision not to breast feed may be difficult. Infant formula should always be used as directed. Proper use of an infant formula is important to the health of the infant. Social and financial implications should be considered when selecting a method of feeding.
The information provided on this website is intended for use by healthcare professionals only. It is a condition of use of this site that you are a healthcare professional within the meaning of regulations within your country of practice. Then denoting below Yes I am/No icons. For Healthcare Professionals based in Australia : It is a condition of use of this site that you are a healthcare professional within the meaning of the Marketing in Australia of Infant Formulas (MAIF) Agreement or the Therapeutic Goods Act.