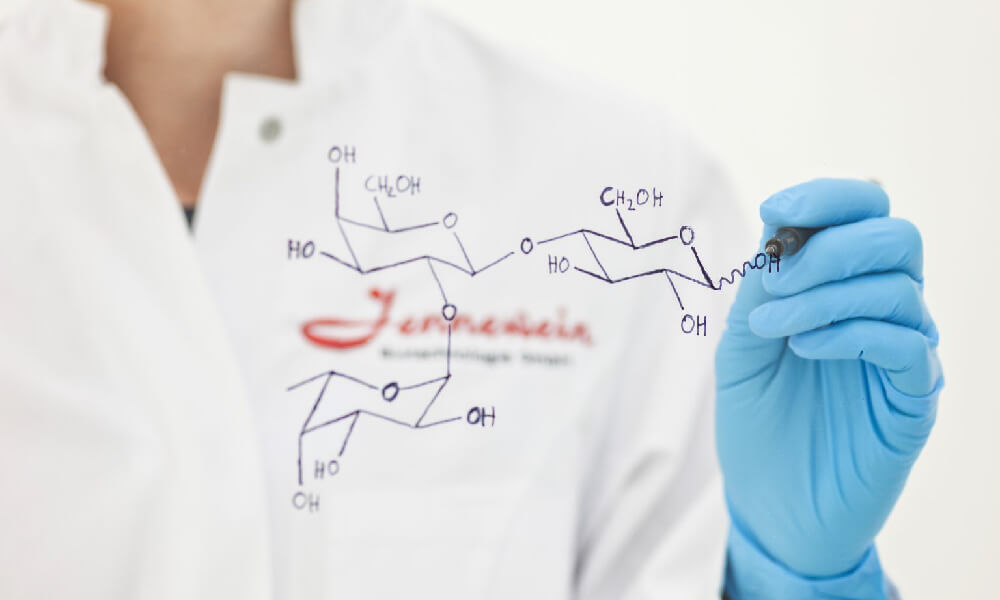
- Breast milk is the optimal nutrition for infants and its composition has been shaped by thousands of years of human evolution in order to provide tailor-made nutrition and protection to the developing neonates. One of the most remarkable component of breast milk is human milk oligosaccharides (HMOs), which are complex carbohydrates that found in mothers breast milk at significant concentrations and with unique structural diversity.
- HMOs are the third most abundant solid component of human milk after lipid and lactose and yet provide no direct nutritional value to the infant.
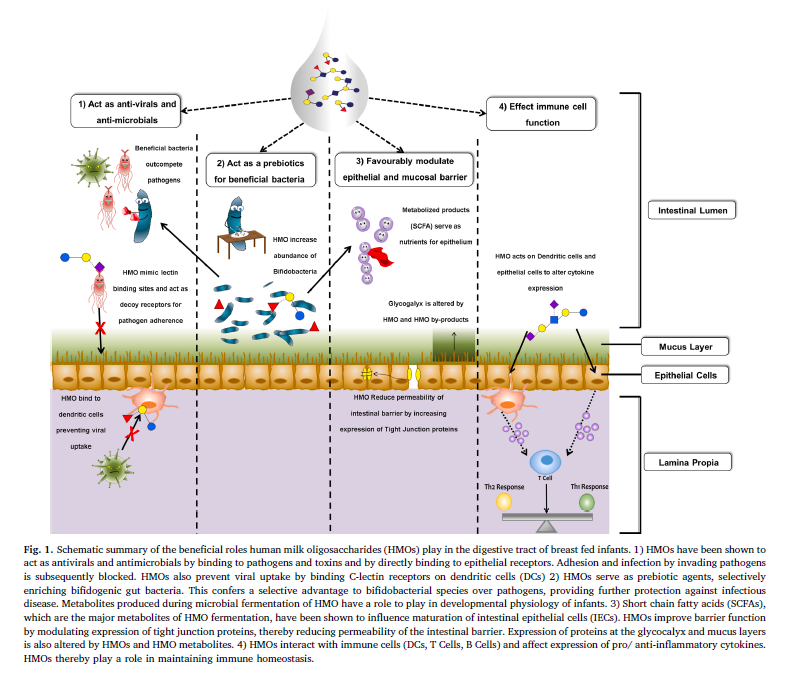
- Recent research has highlighted the various functional roles of HMOs in infant development, including as prebiotics by promoting growth of beneficial intestinal bacteria thereby generating short-chain fatty acids which are very important for gut health. HMOs also play a central role in the development of the neonatal immune system by promoting healthy microbial diversity, preventing pathogen attachment, stimulating maturation of intestinal epithelial surface and by modulating the immune cells.
The concentration of HMOs varies based on the breastmilk phases with the highest level in colostrum (20 g/L) and decrease across lactation, with 16 g/L detected in 30-day mature milk. HMOs are structurally and biologically diverse group of complex indigestible sugars. Currently, there are 150 – 200 structures of HMOs that have been identified.
Human milk contains HMOs in the level of 100 – 1000 times higher than in the milk of any domesticated farm animals. This is the reason of the absence of HMOs in the infant formula milk, until recently some infant formula being added with HMOs. The commercial availability of these HMOs has allowed for a deeper appreciation of the functions of HMOs, many of which are believed to be exerted through interactions with the gut microbiota.
It was noted at the beginning of the 20th century, that infant mortality rates were lower in breastfed infants when compared to those of bottle-fed infants, with decreased occurrences of infectious disease. The scientists noticed variances in the bacterial composition between breast-fed and bottle-fed infant feces, substantiating claims that feeding regime and composition of intestinal bacteria were linked to infant health. In 1954, the bifidus factor in human milk responsible for enrichment of bacteria in breastfed infants was identified as HMOs.
Data from ex vivo and in vivo studies, including recent intervention studies in humans, highlight the importance of HMOs in the infant gastrointestinal tract as summarized well in Fig.1. Many infant gut bacteria, particularly members of the genus Bifidobacterium, possess the genetically encoded ability to consume HMOs, providing rationale for their predominance in the gut of breast-fed infants. HMOs serve as growth factor for beneficial bacteria by supplying metabolic substrates necessary for beneficial bacteria to thrive. By acting as prebiotic agents, HMOs confer a selective advantage to beneficial microbes over pathogens, thereby providing protection to the infant from infectious diseases.
HMO-mediated protection has also been described for many viruses such as rotaviruses or noroviruses, which are some of the leading causes of gastroenteritis outbreaks. HMOs function as antivirals by mimicking receptor sites and blocking entry into the cell, but also by preventing viral replication within the cell. One of the earliest studies demonstrating the potential of HMOs to reduce infectivity of Norovirus found that human milk blocked genogroup I genotype 1 (GI.1) and genogroup II genotype 4 (GII.4) binding to saliva.
The intestinal epithelium lining in the small intestine and colon is considered the first line of defense in innate immunity, since it acts as a physical, rate-limiting barrier between the gut lumen and the circulatory system. Permeability of the epithelium is dependent primarily on the structure and expression of tight junctions. Researchers have demonstrated that HMOs promote tight junction protein expression (Fig. 1) and increase differentiation along the cryptvillus axis.
In addition to their role in an infant’s intestinal lumen, HMOs also contribute to the development and physiological functions of other organs and systemic cell systems. While the vast majority of HMOs pass undigested into the colon, about 1 – 5% HMOs is absorbed from the gastro-intestinal tract to the circulatory system. Studies have shown that HMOs modulate immune cell-cell interactions, thereby facilitating maintenance of balanced inflammatory cell responses (Fig. 1).

Are you a Healthcare Professional?
Important Notice and Declaration
Breast milk is best for babies. Professional advice should be followed before using an infant formula. Introducing partial bottle feeding could negatively affect breast feeding. Good maternal nutrition is important for breast feeding and reversing a decision not to breast feed may be difficult. Infant formula should always be used as directed. Proper use of an infant formula is important to the health of the infant. Social and financial implications should be considered when selecting a method of feeding.
The information provided on this website is intended for use by healthcare professionals only. It is a condition of use of this site that you are a healthcare professional within the meaning of regulations within your country of practice. Then denoting below Yes I am/No icons. For Healthcare Professionals based in Australia : It is a condition of use of this site that you are a healthcare professional within the meaning of the Marketing in Australia of Infant Formulas (MAIF) Agreement or the Therapeutic Goods Act.